Currently, some customers ask for test method of PQQ Disodium from us, now we’d like to share this info as below :
TEST METHOD OF PQQ DISODIUM

Product name: Pyrroloquinoline Quinone disodium salt, PQQ Disodium
CAS number: 122628-50-6
Structural formula: 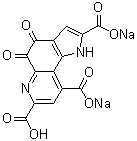
4, 5-dihydro -4,5-dioxo-1H-pyrrolo [2,3-f] quinoline-2,7,9-Tricarboxylic acid disodium
Molecular formula: C14H4N2Na2O8
Molecular weight: 374.17
This product use area normalization method for calculating the purity and the C14H4N2Na2O8 content should be no less than 98.0%.
- Character
This product is red to reddish-brown powder.
- Check
- Check alcohol solvents residual
Precisely take this product 0.1g and put it into 10ml volumetric flask and add with 0.1mol/L sodium hydroxide solution to dissolve and dilute to the scale, shake it. This is regarded as the test solution.
Precisely take ethanol 50mg and put it into 100ml volumetric flask and add with 0.1mol/L sodium hydroxide solution to dissolve and dilute to the scale, shake it. This is regarded as the reference solution.
According to the residues solvent determination method (China Pharmacopoeia 2010 version Part II Appendix VIII p the third method), capillary color spectrum column (30m × 0.53mm, ø = 3 μ m).
Take 6% Cyanide propyl phenyl- 94% dimethyl polysiloxane as the stationary liquid; Starting temperature is 40 ℃, maintain 5 minutes, then rise to 160 ℃ at the speed of 20℃/minute, maintain 3 minutes. The temperature for injection port is 200 ℃; detection device temperature is 250 ℃; flame ionization detector (FID).
Precisely measure 1ul test solution and 1ul reference solution, respectively injected them into the gas chromatograph and record the chromatogram. Calculate the peak area by the external standard method, should not be more than 0.5%.
- Check moisture
Precisely measure this product 1.0g. Calculate the moisture by method of the loss on drying (China Pharmacopoeia 2010 version Part II Appendix VIII L). Drying the product at the temperature of 130 ℃ for 4 hours to the constant weight. The water should not be more than 12.0%.
- Content determination
According to High-performance liquid chromatography (China Pharmacopoeia 2010 version Part II Appendix V D)
Chromatographic conditions and system suitability test:
Column: YMC-C18, 250 × 4.6,
Use Acetonitrile: 0.1mol/L and the Monopotassium phosphate =12:88 (use phosphoric acid to adjust pH to 2.0) as the mobile phase,
Flow rate : 1.0mL/min,
Detection wave length: 249nm,
Column temperature: 35 ℃
number of theoretical plate should be no less than 3000.
Measuring method : Precisely measure this product 30mg, put it into 100ml volumetric flask, then add the mobile phase to dissolve and dilute to scale, shake it. This is regard as the test solution.
Injection volume : 10 ml, injected into liquid chromatograph and record the chromatogram.
This product use area normalization method for calculating the purity and the C14H4N2Na2O8 content should be no less than 98.0%.


